Division MYZOZOA
Class DINOPHYCEAE
Order AMPHIDINIALES
Amphidinium crassum, Amphidinium longum, Amphidinium sphenoides.
Order DINOPHYSALES
Dinophysis acuminata, Dinophysis norvegica, Phalacroma rotundatum, Dinophysis rotundata.
Order GONYAULACALES
cf. Alexandrium minutum, Alexandrium ostenfeldii, Amylax triacantha, Tripos muelleri, Ceratium tripos, Dissodinium pseudolunula, Micracanthodinium setiferum, Sourniaea diacantha, syn. Gonyaulax verior, Peridiniella catenata.
Order GYMNODINIALES
Akashiwo sanquinea, Gymnodinium sp., Gyrodinium spirale, Gyrodinium sp., Lebouridinium glaucum, Kotodinium glaucum, cf. Nematodinium armatum, Proterythropsis vigilans, Gymnodinium corollarium.
Order PERIDINIALES
Diplopsalis, Heterocapsa rotundata, Kryptoperidinium triquetrum, syn. Heterocapsa triquetra, Oblea rotunda complex, Preperidinium meunieri, Protoperidinium bipes, Protoperidinium brevipes, Protoperidinium cf. granii, Protoperidinium sp.
Order PROROCENTRALES
Prorocentrum cordatum, Prorocentrum minimum.
Order SUESSIALES
Biecheleria baltica.
Order THORACOSPHAERALES
Pfiesteria sp.
Order TORODINIALES
Kapelodinium vestifici.
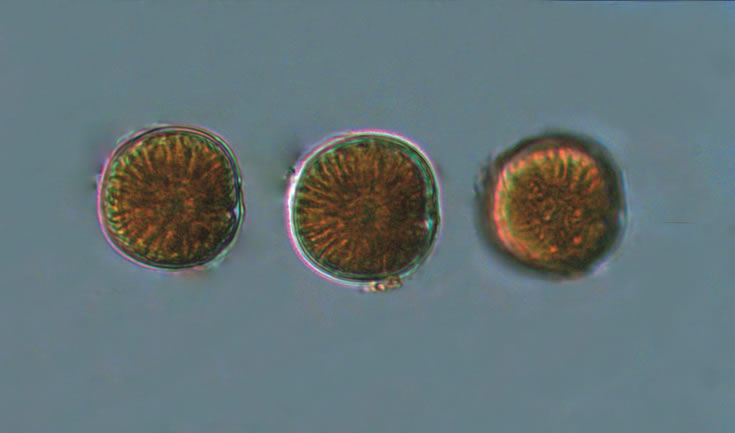
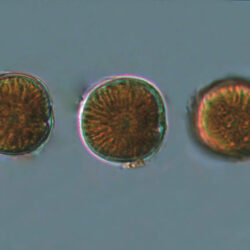
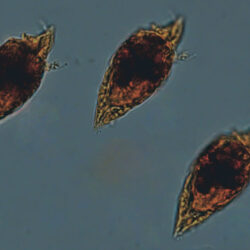
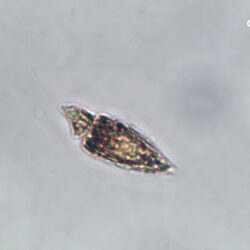
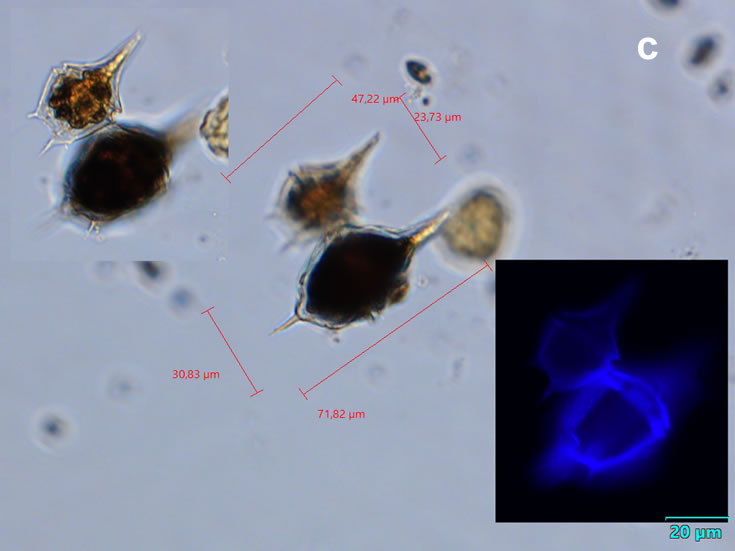
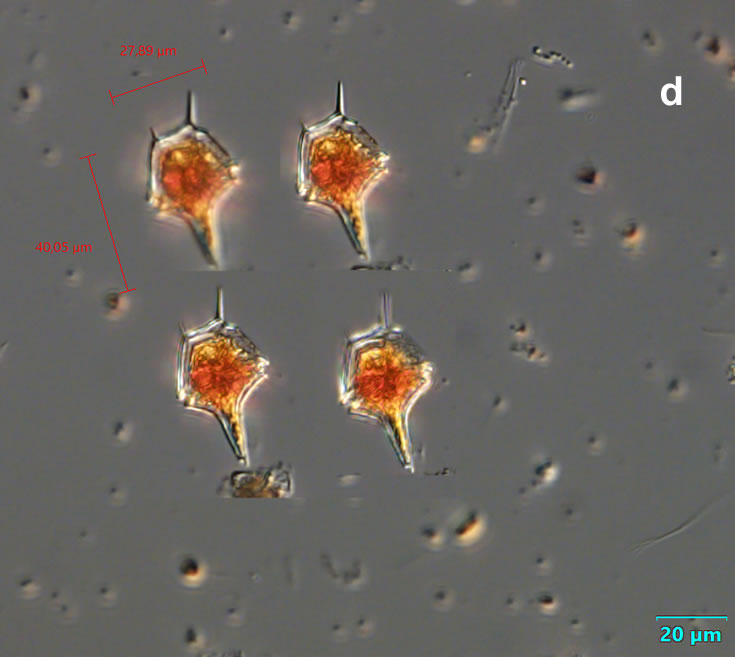
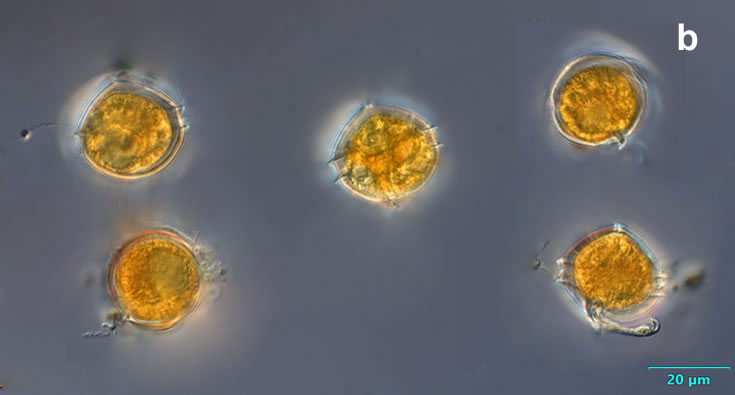
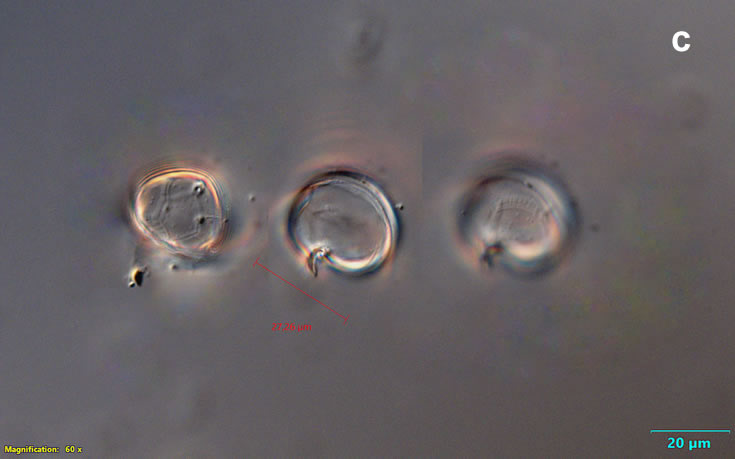
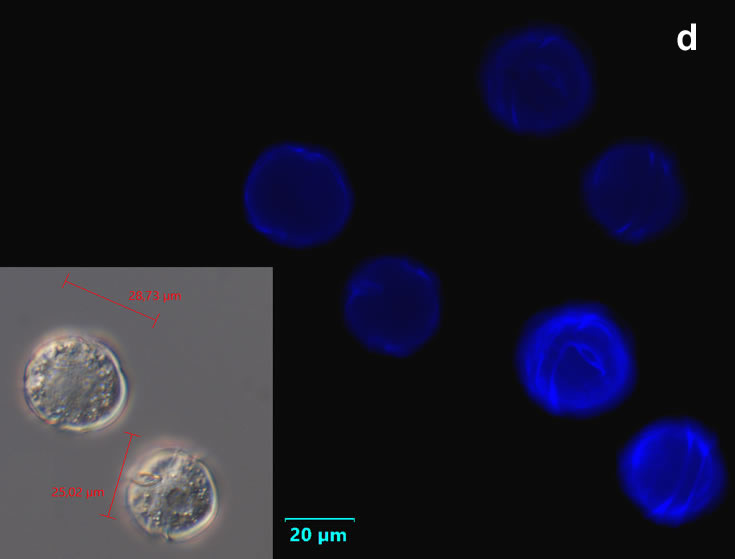
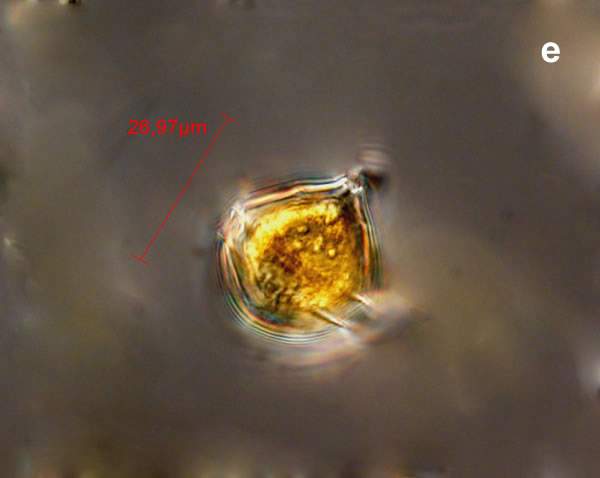
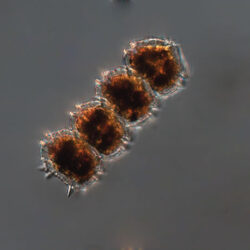
Even though Alexandrium ostenfeldii was recorded in the Baltic Sea as early as 1937 by Schiller off the coast of Germany, and in the Gulf of Riga since 1953, it was not observed on the Polish coast until 2001, when it appeared in bloom quantities near Chałupy, causing the sea to glow. The second such large bloom occurred in 2012 near Rewa. In addition, large blooms of this species were observed in two other places in the Baltic Sea: off the coast of the Swedish island of Öland in 1997, and in the summers of 2003 and 2004 on the Finnish coast near Åland. It also occurs in other seas and oceans, but always in the temperate climate zone. The average annual biomass does not exceed 50 µg/l and abundance does not exceed 2000 cells/l, only in 1991, 1999, 2001 and 2009 the biomass and number were higher (respectively: 206, 264, 159 and 78 µg/l and 16000, 12000, 7000, 4000 cells/l)
OCCURRENCE: late spring, summer, autumn video nr 1 – Oblea rotunda complex In the 1980s Peridiniella catenata reached a maximum in May, while in later years in April, which may be a result of climate change. The highest abundance and biomass were in the 1980s and 1990s when P. catenata blooms were observed (up to 4 000 µg/l ), while after 2000 they were 10 times lower (not exceeding 300 µg/l), which may also be related to the appearance of other dinoflagellates (of the genera Biecheleria, Apocalathium, Scrippsiella, Gymnodinium, Heterocapsa) in the phytoplankton assemblage of the southern Baltic Sea, previously not present in such high numbers. It is believed that the appearance of these competing genera of dinoflagellates is also related to climate change, with them especially abundant after warm winters. video nr 2 – Peridiniella catenata, February 2020, Puck Bay OCCURRENCE: late summer – autumn, with maximum in September Prorocentrum cordatum is considered an invasive alien species in the Baltic Sea *. It appeared here in the 1980s, while in the late 1990s and early 2000s it formed dangerous, very intense blooms (also called red tides).Alexandrium ostenfeldii, (Paulsen) Balech & Tangen, 1985
NUTRITION: autotrophic
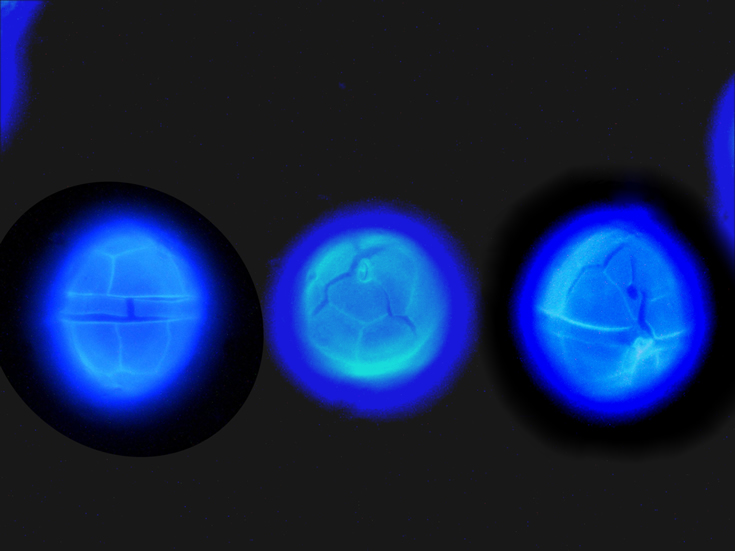
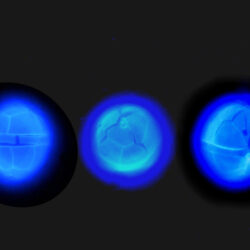
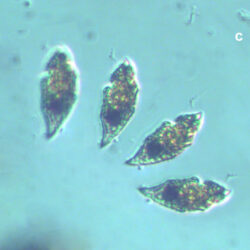
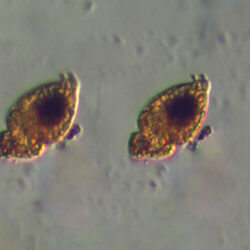
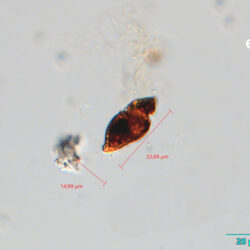
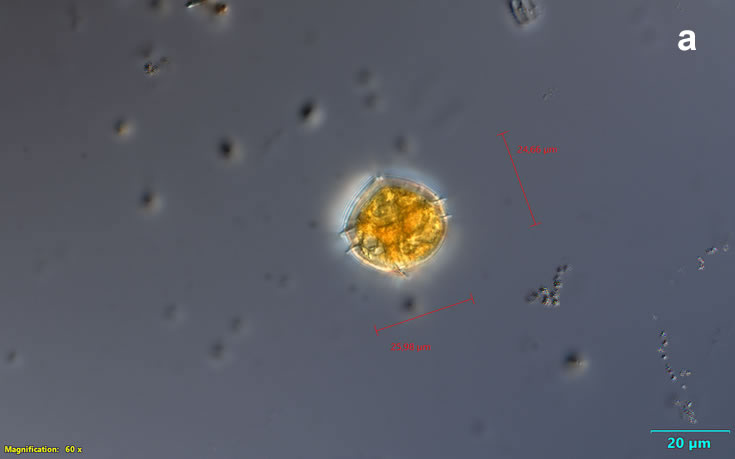
ENVIRONMENTAL IMPACT: toxic species, produces a highly toxic saxitoxin belonging to the group of neurotoxins, which can cause fatal respiratory paralysis in mammals (PST – Paralytic Shellfish Toxins). Although it usually occurs in small concentrations in the Bay of Puck and the southern Baltic Sea, in favorable conditions it causes blooms that induce sea glow at night when the water is agitated by a sudden movement, e.g. by an object thrown into it or strong waves. This causes blue bioluminescence of cells – https://www.polskieradio.pl/23/266/Artykul/673841,Zatoka-Pucka-swieci-w-nocy-Odpowiedzialne-bruzdnice
CELL SIZE: 32-50 x 32-55 µm
LIFE FORM: solitary
ECOLOGY: marine species
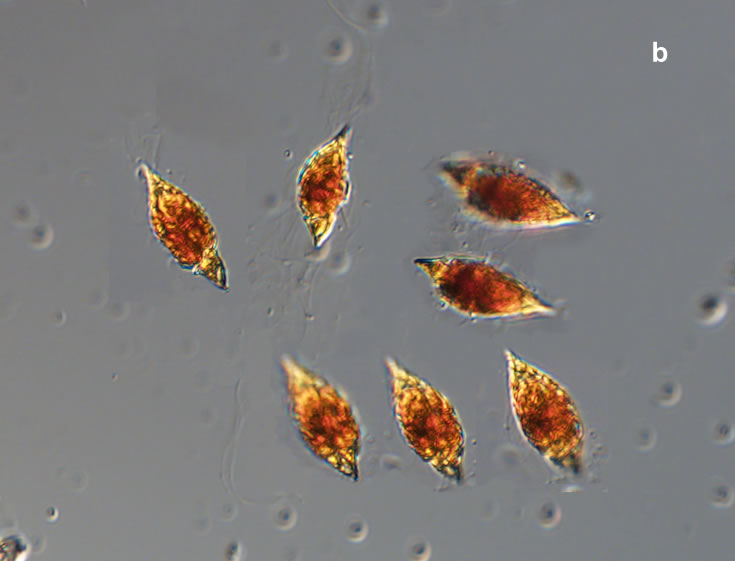
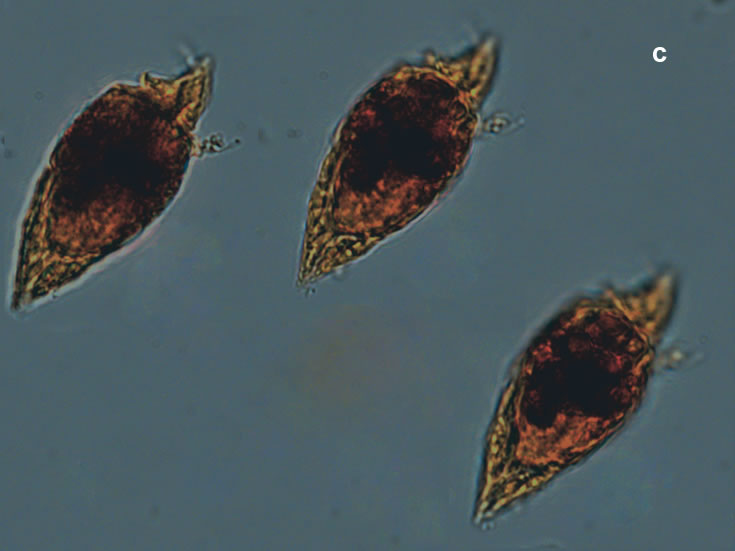
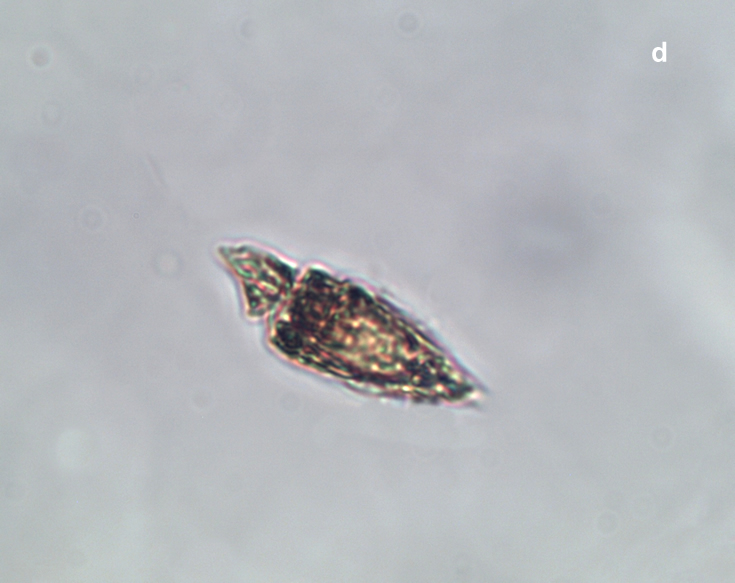
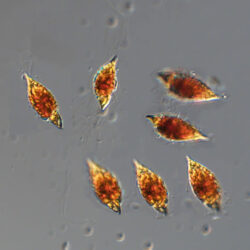
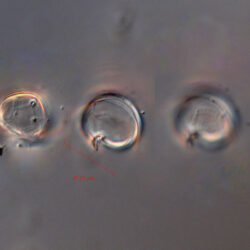
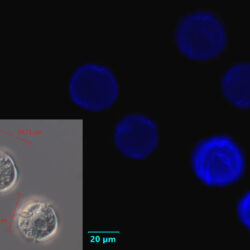
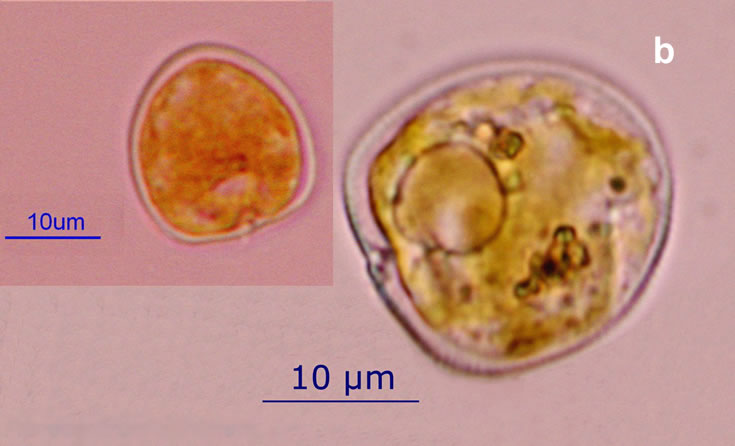
PRESERVATION: living cells – Rewa, end of August 2012 (a);
epifluorescence microscopy with use of Calcofluor, living cells – Rewa, end of August 2012 (b);
cells preserved with Lugol – Chałupy, August 2001 (c,d).
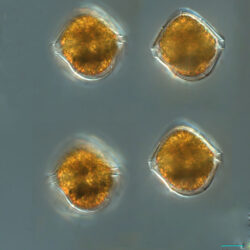
Amphidinium crassum, Lohmann 1908
NUTRITION: heterotrophic
ENVIRONMENTAL IMPACT: friendly, not t known to be harmful
CELL SIZE: length 15-30 µm, width 8-20 µm (cel shape – flattened elipsoid)
LIFE FORM: solitary
ECOLOGY: marine, brackish species
PRESERVATION: Autumn 2008, Lugol (a);
July 2020, Puck Bay, Lugol (b);
August 2020, Puck Bay, Lugol (c).
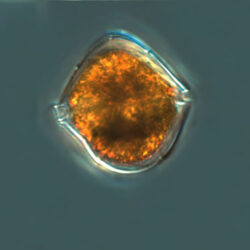
Amphidinium sphenoides, Wülff 1916
NUTRITION: heterotrophic
ENVIRONMENTAL IMPACT: friendly, not t known to be harmful
CELL SIZE: length 30-40 µm, width 14-15µm
LIFE FORM: solitary
ECOLOGY: marine
PRESERVATION: April 2017, Lugol (a);
April 2020, Puck Bay, Lugol (b);
March 2020, Puck Bay, Lugol (c);
April 2015, southern Baltic Sea, Lugol (d).
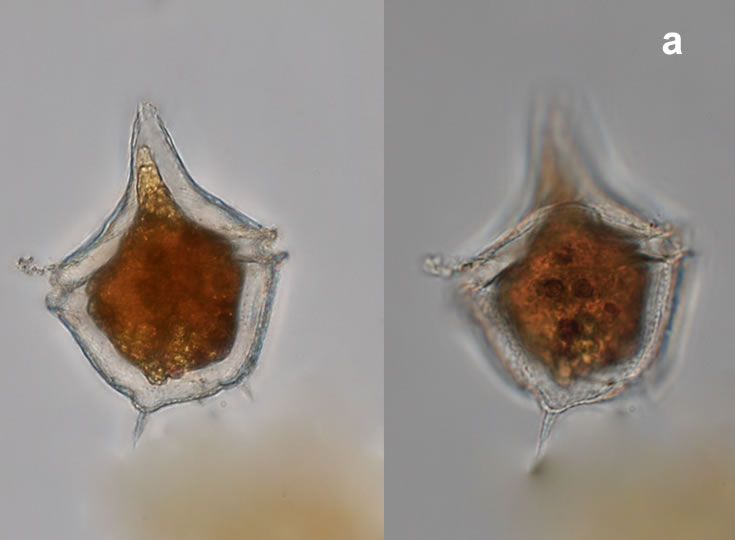
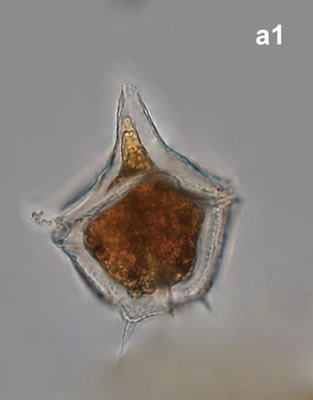
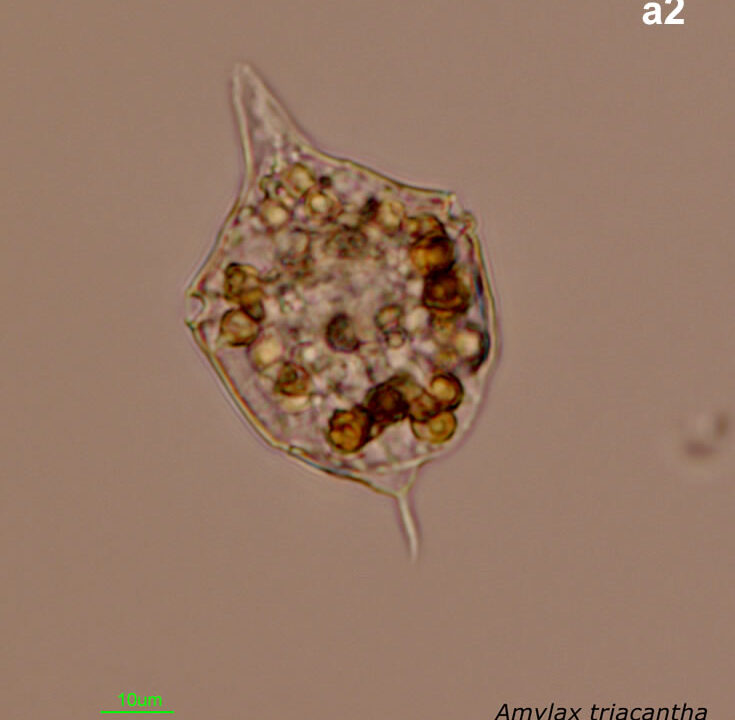
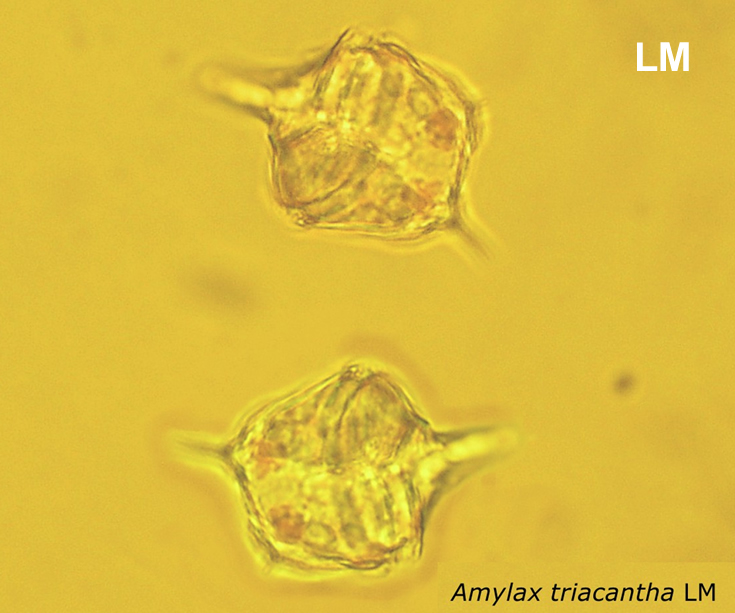
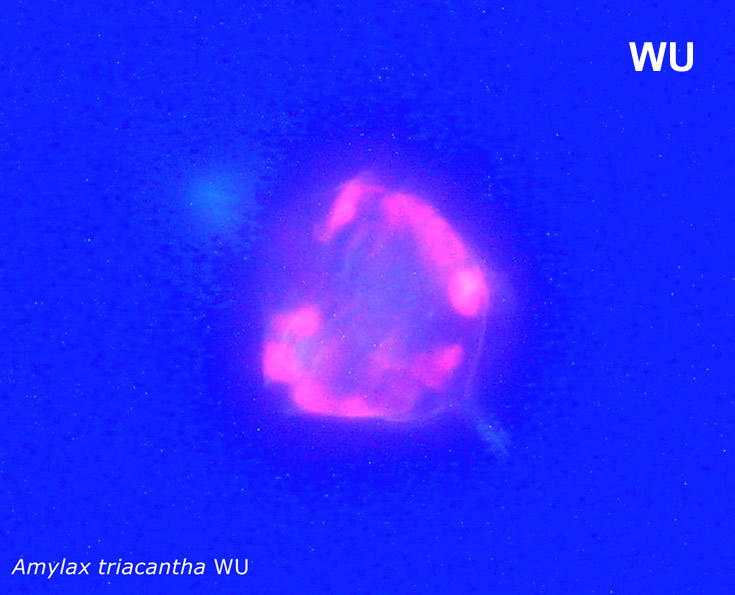
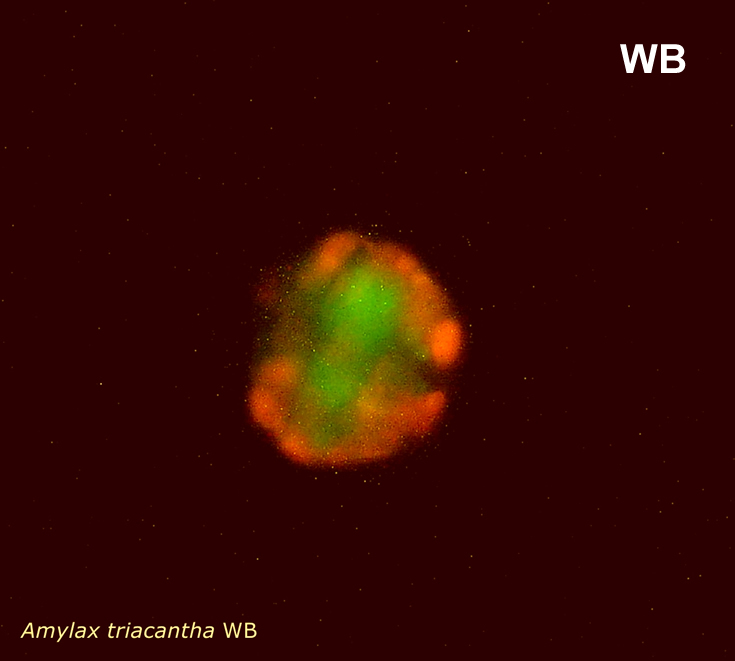
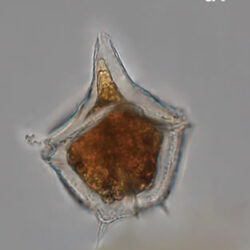
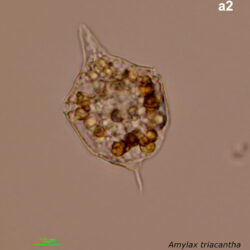
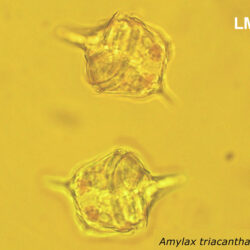
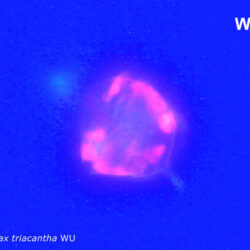
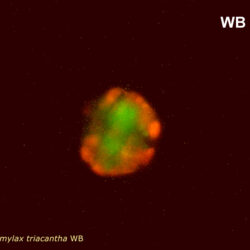
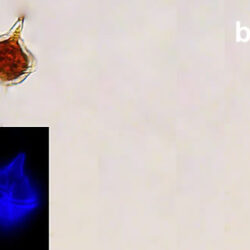
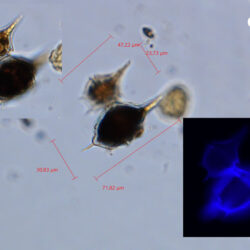
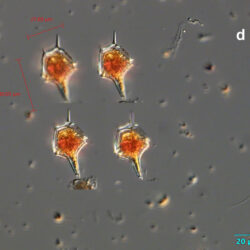
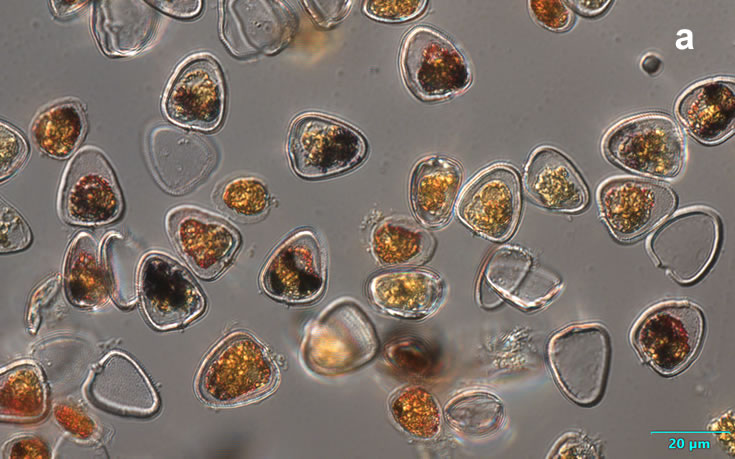
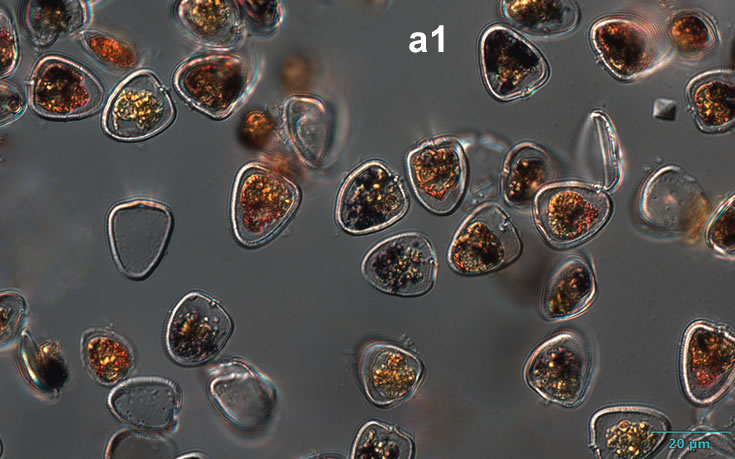
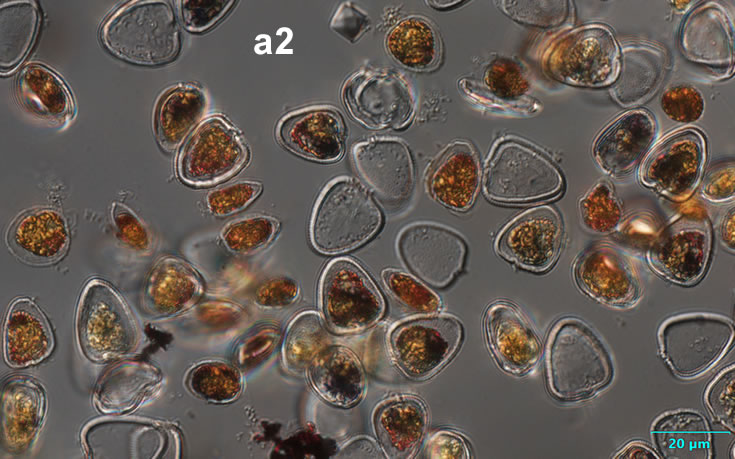
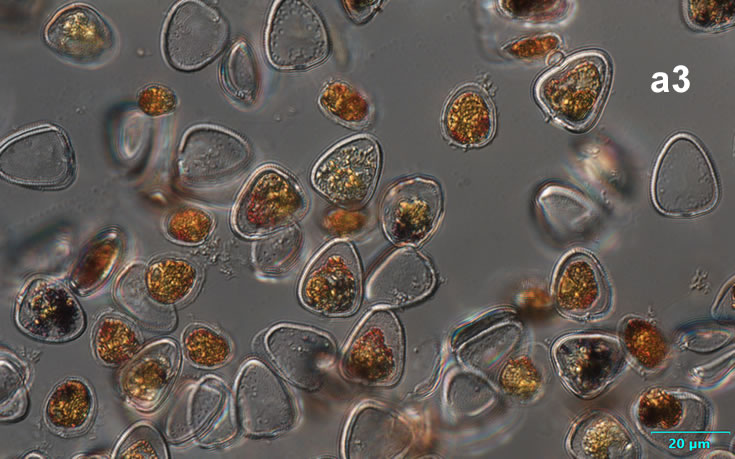
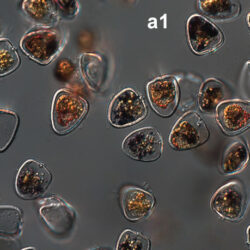
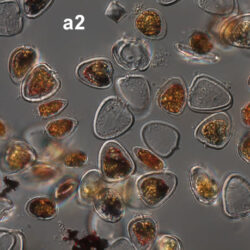
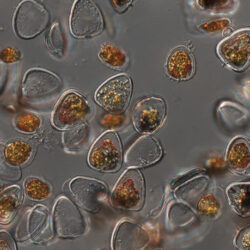
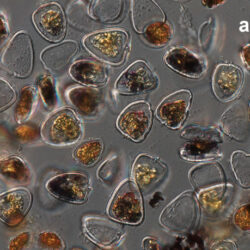
Amylax triacantha, (Jörgensen) Sournia 1984
NUTRITION: autotrophic
ENVIRONMENTAL IMPACT: friendly, not known to be toxic
CELL SIZE: length 35-65 µm, width 34-40 µm
LIFE FORM: solitary
ECOLOGY: marine species
PRESERVATION: April 2008, southern Baltic Sea, Lugol the same cell at different focus (a);
April 2008, southern Baltic Sea, Lugol (a1);
April 2008, southern Baltic Sea, Lugol, living cell (a2);
May 2007, southern Baltic Sea, living cell in light microscopy(LM);
epifluorescence microscopy (WU) with Calco Fluor White M2R fluorescent brighter elicited a blue fluorescence of a background and a cellulose theca, and a pinky-red fluorescence of chlorophyll-a.; (WB) green autofluorescence of the cytoplasm (carotenoids probably), and red autofluorescence of chlorophyll-a;
April 2020_Puck Bay, Lugol with addition of Calco Fluor White M2R (b);
April 2020, Puck Bay, Lugol with addition of Calco Fluor White M2R (c);
May 2020, Puck Bay, Lugol (d);
May 2022, Mechelinki, Lugol: (a), living cell (b), red autofluorescence of chlorophyll-a (c).
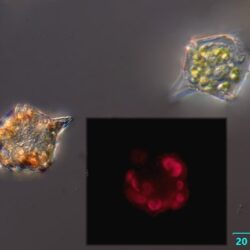
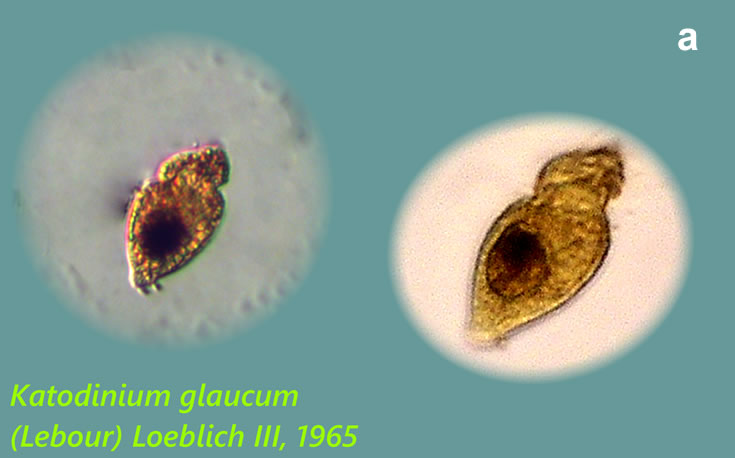
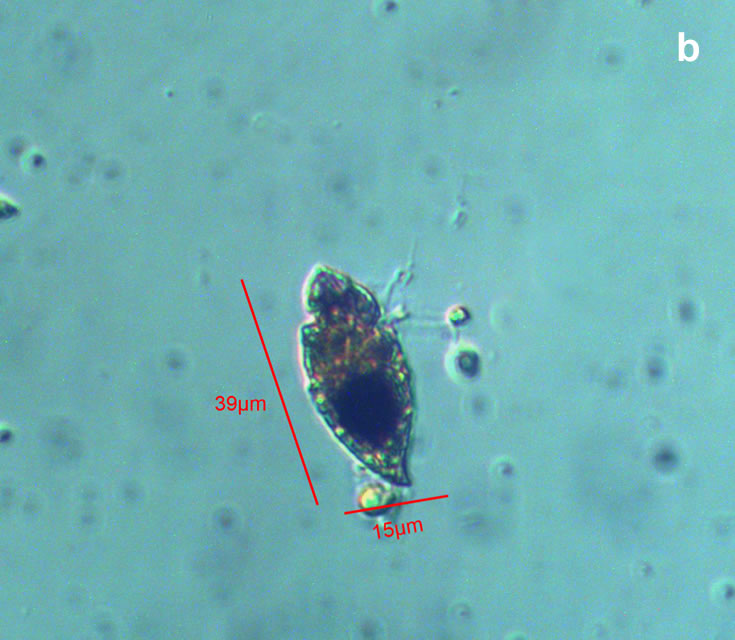
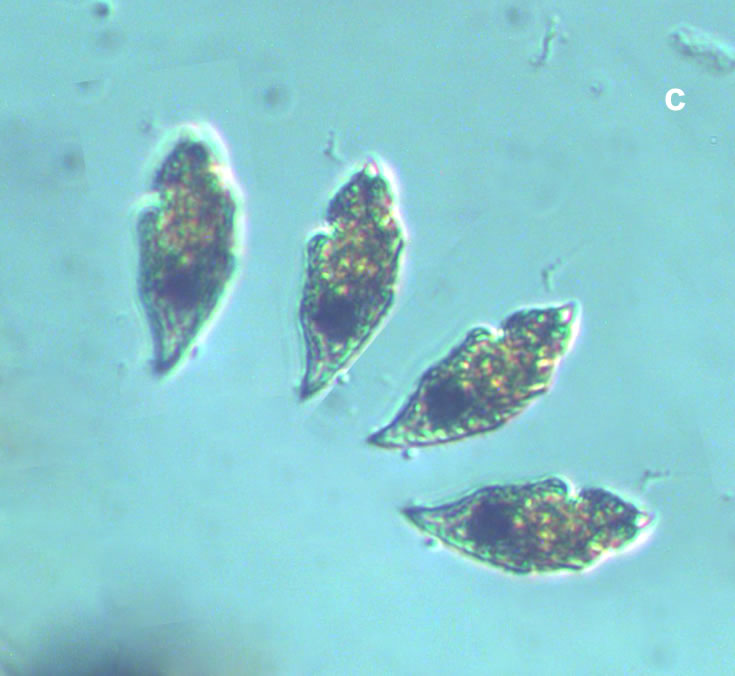
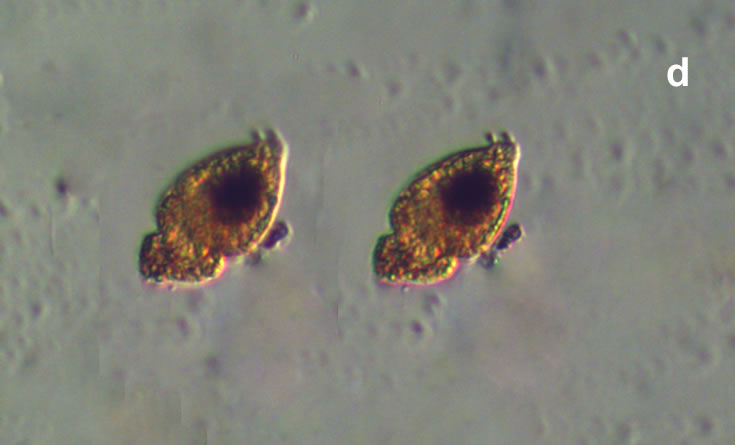
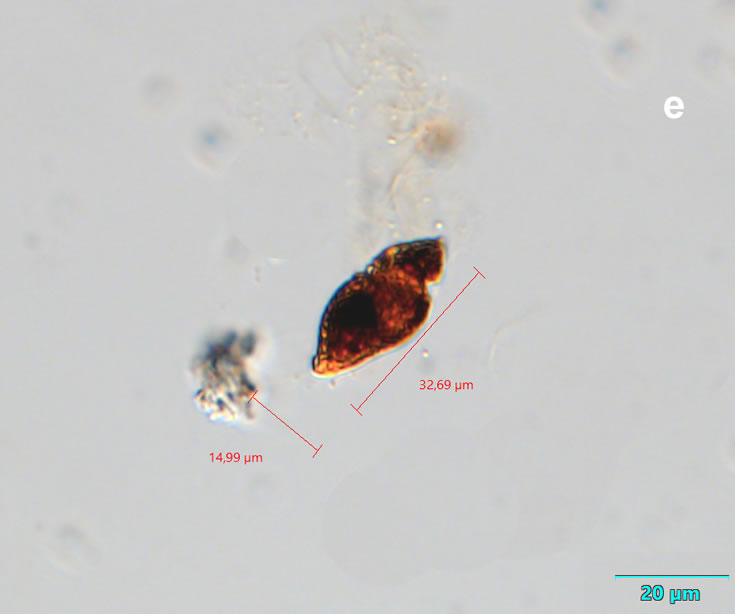
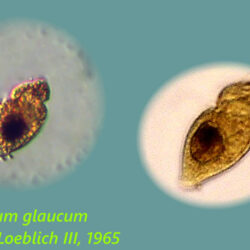
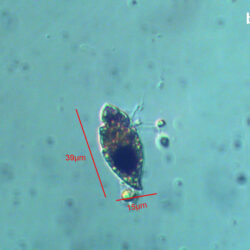
Lebouridinium glaucum (Lebour) 2016; syn. Katodinium glaucum (Lebour) Loeblich III 1965
NUTRITION: heterotrophic
ENVIRONMENTAL IMPACT: not known to be harmful
CELL SIZE: 14-30 x 22-62 µm
LIFE FORM: solitary, non thecate
ECOLOGY: marine species.
PRESERVATION: Maj 2009, southern Baltic Sea, Lugol (a);
Maj 2012, Bornholm Basin, Lugol (b);
Maj 2012, Bornholm Basin, Lugol – one cell at different focus (c);
April 2017, southern Baltic Sea, Lugol – one cell at different focus (d);
Maj 2020, Puck Bay, Lugol (e).
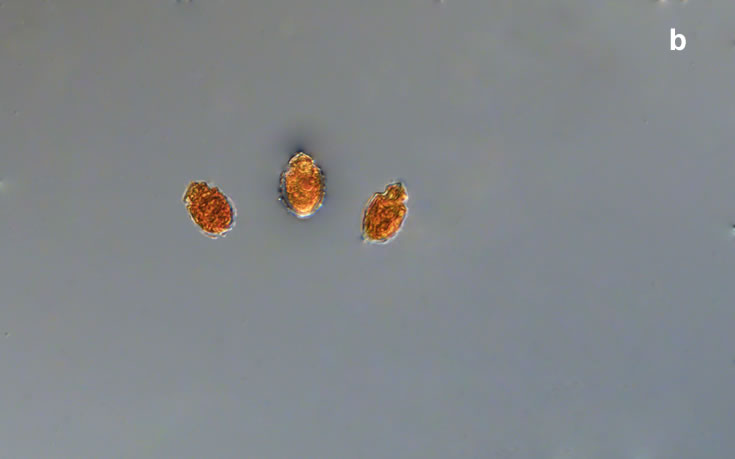
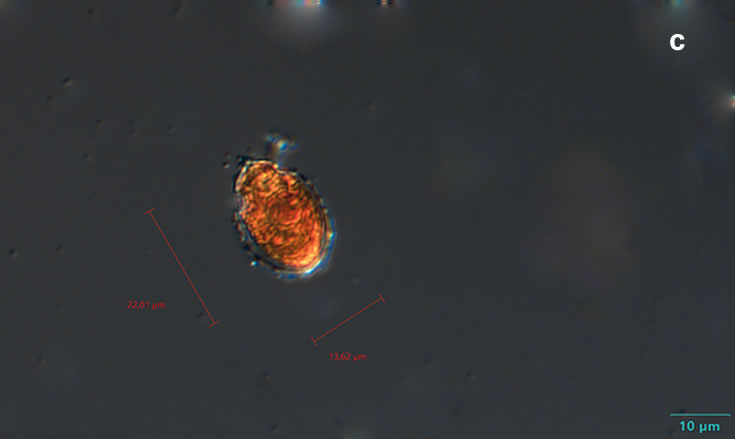
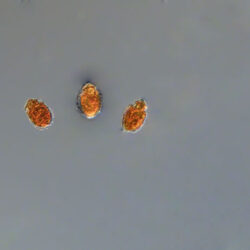
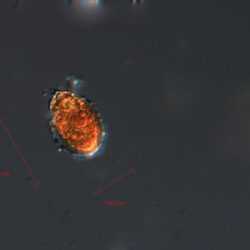
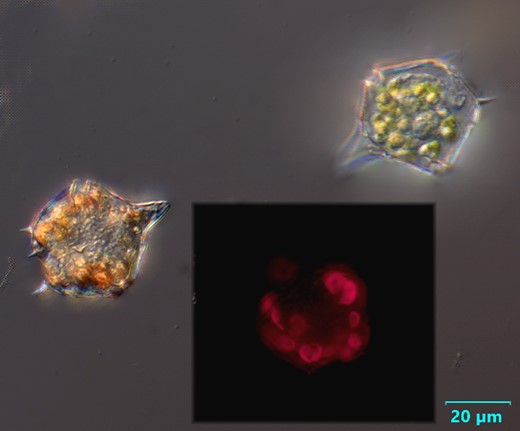
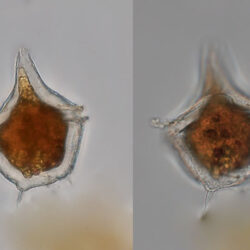
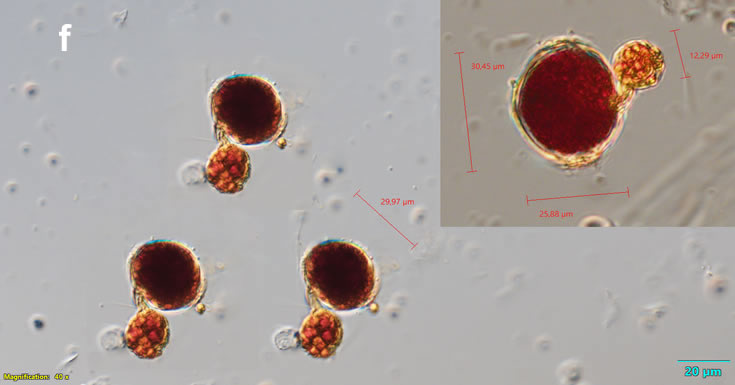
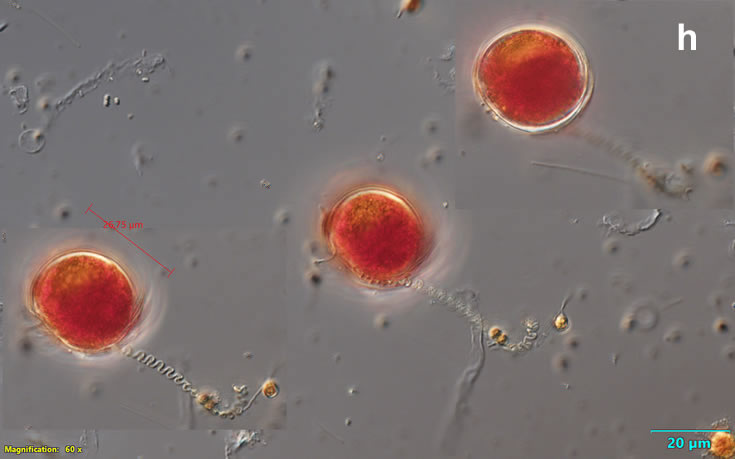
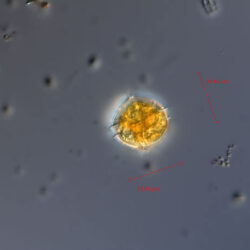
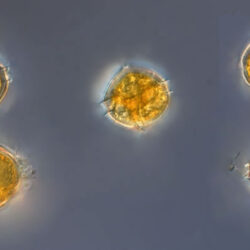
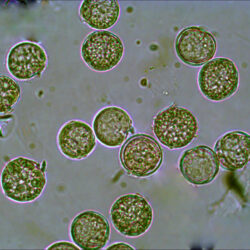
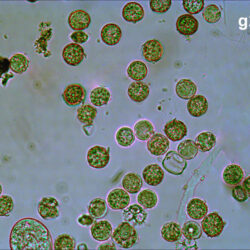
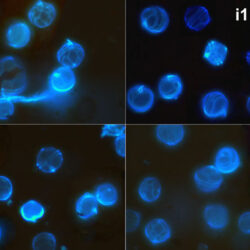
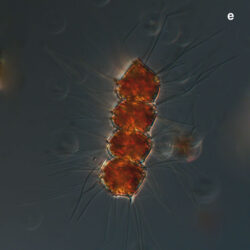
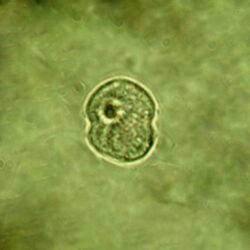
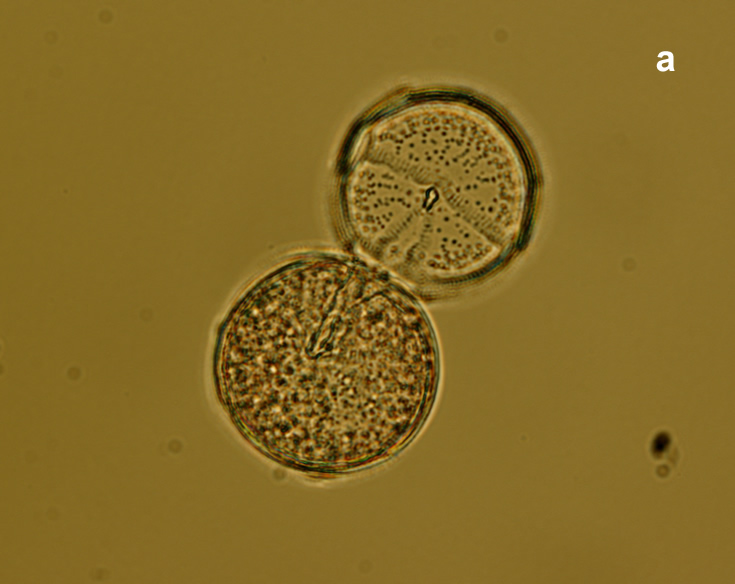
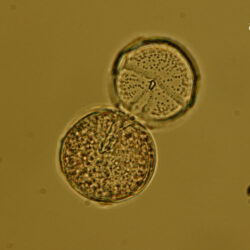
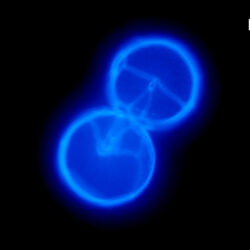
Oblea rotunda complex
NUTRITION: heterotrophic
ENVIRONMENTAL IMPACT: not known to be harmful
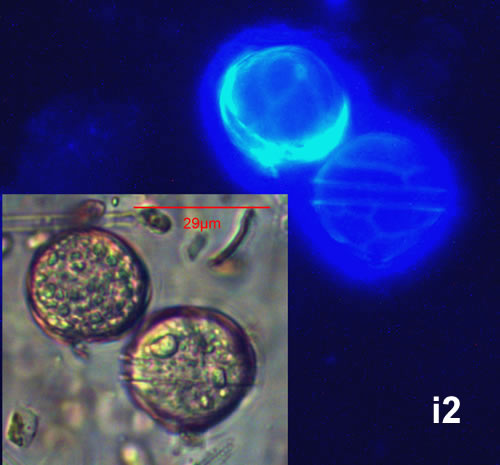
CELL SIZE: 22-42 µm
LIFE FORM: solitary
ECOLOGY: marine species
PRESERVATION: May 2022, Mechelinki, Lugol (a);
May 2022, Mechelinki, Lugol – few cells (b);
May 2022, Mechelinki, Lugol – empty theca (c);
May 2022, Mechelinki, Lugol – light microscopy and epifluorescence microscopy with use of Calcofluor (d);
September 2020, southern Baltic Sea, Lugol (e);
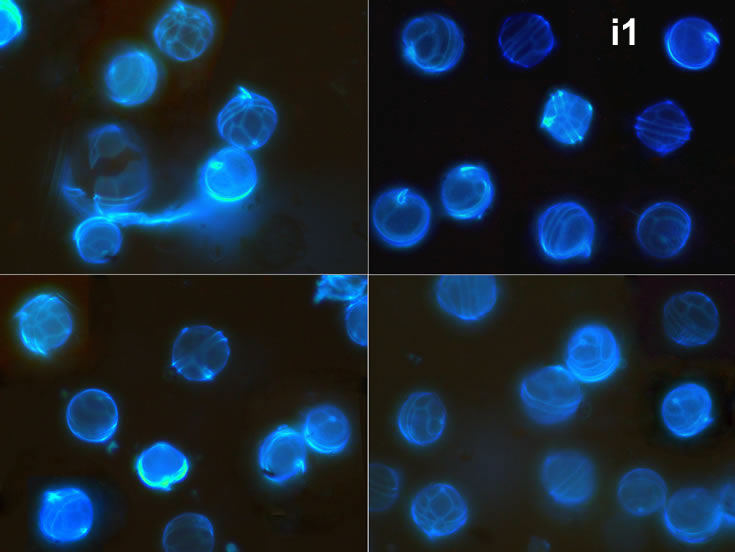
May 2022, culture Oblea catching food particles (f);
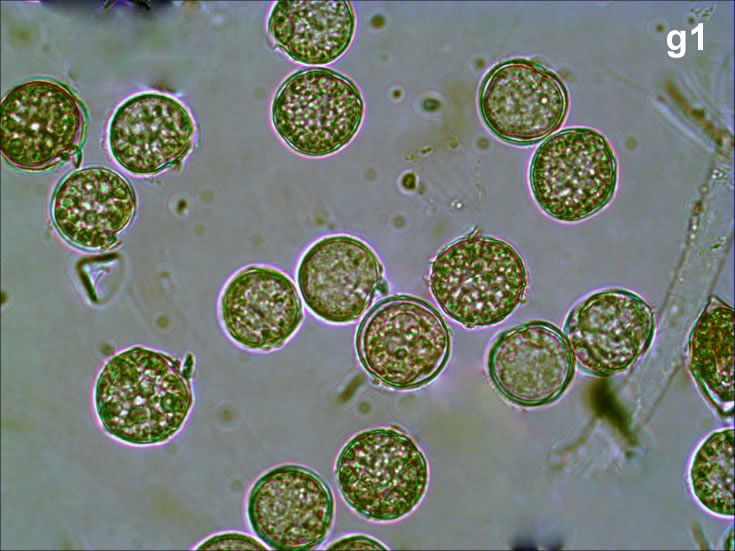
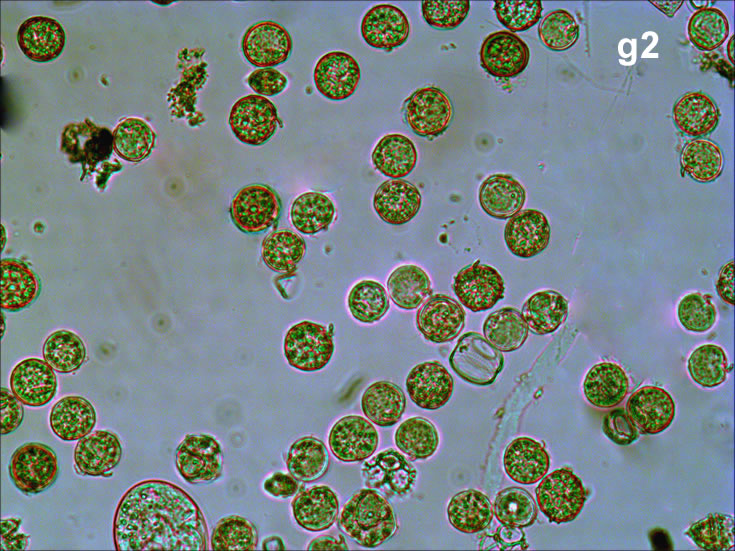
Oblea rotunda complex – mass occurrence near Gdynia boulevard (g1, g2);
May 2022, culture one cell at different focus (h)
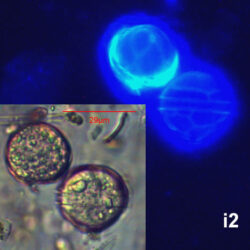
May 2013, mass occurrence near Sopot pier, epifluorescence microscopy with use of Calcofluor (i1, i2);
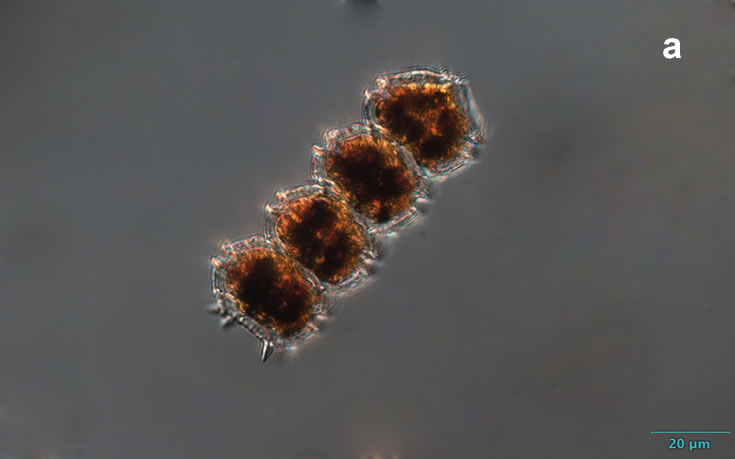
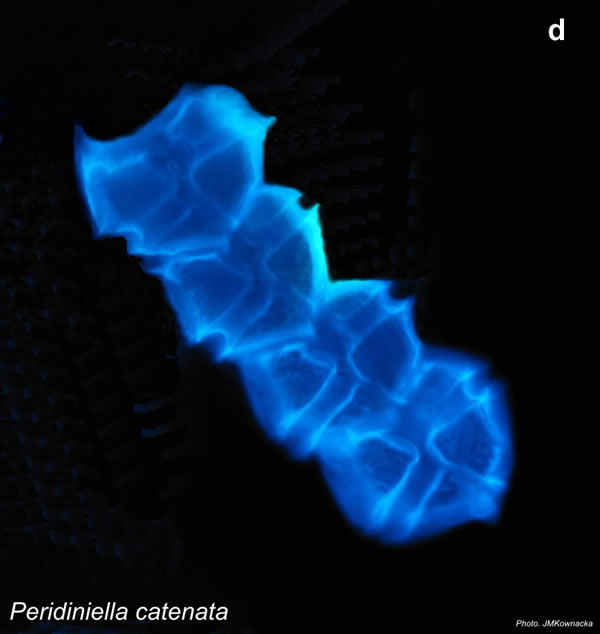
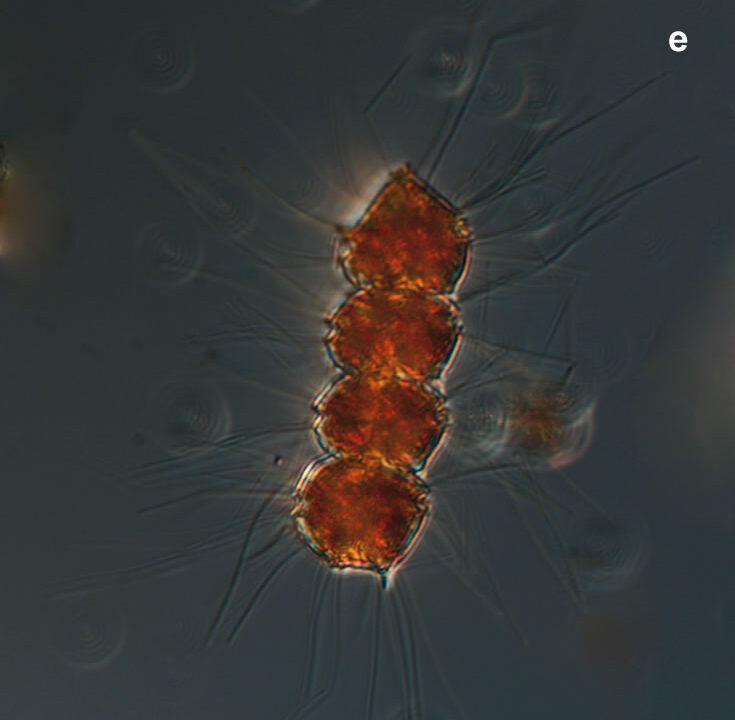
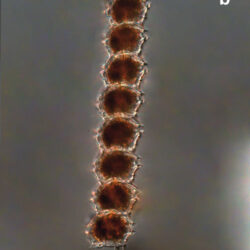
Peridiniella catenata, (Levander) Balech 1977
NUTRITION: autotrophic
ENVIRONMENTAL IMPACT: friendly
CELL SIZE: 20-40 µm
LIFE FORM: solitary cell and chains with different number of cells, usually 2, 4, 8, 16
ECOLOGY: marine species
PRESERVATION: April 2006, Gdańsk Deep, Lugol – chain of 4 cells (a);
April 2006, Gdańsk Deep, Lugol – chain of 8 cells (b);
April 2008, living cells (c);
April 2010_living cells, epifluorescence microscopy with use of Calcofluor (d);
February 2020, Lugol – chain of old cells (e).
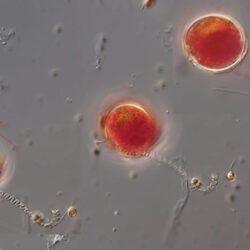
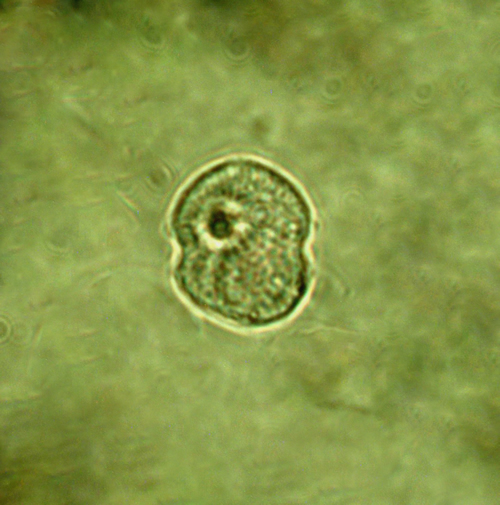
Pfiesteria, Steidinger et Burkholder 1996
NUTRITION: heterotrophic
ENVIRONMENTAL IMPACT: potentially extremely toxic
CELL SIZE: 15 µm
LIFE FORM: solitary
ECOLOGY: marine species
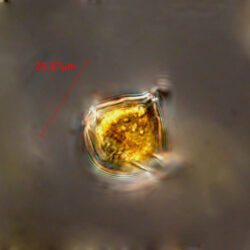
PRESERVATION: June 2012, Gdynia boulevard, living cel, light microscopy
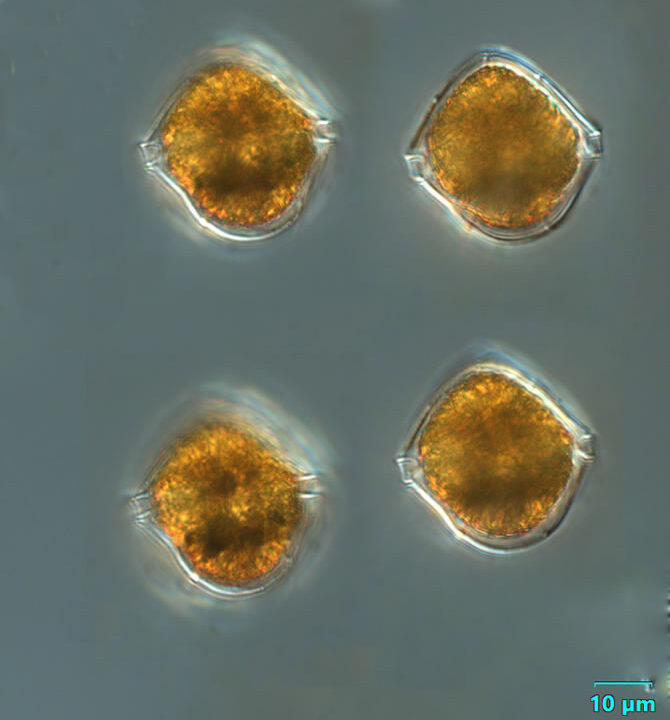
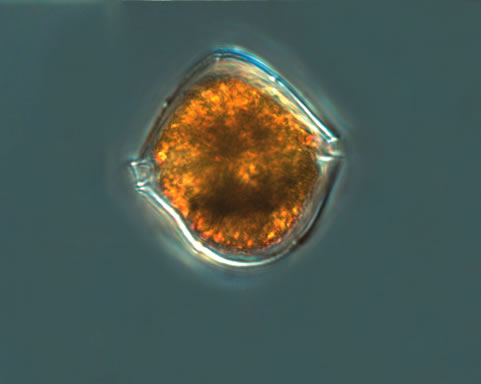
Preperidinium meunieri (Pavillard) Elbrächter 1993
NUTRITION: heterotrophic
ENVIRONMENTAL IMPACT: not known to be harmful
CELL SIZE: 28-38µm x 48-55µm
LIFE FORM: solitary
ECOLOGY: marine species
PRESERVATION: August 2008, living cell, light microscop (LM), (a);
August 2008, living cell epifluorescence microscopy with use of Calcofluor (WU), (b).
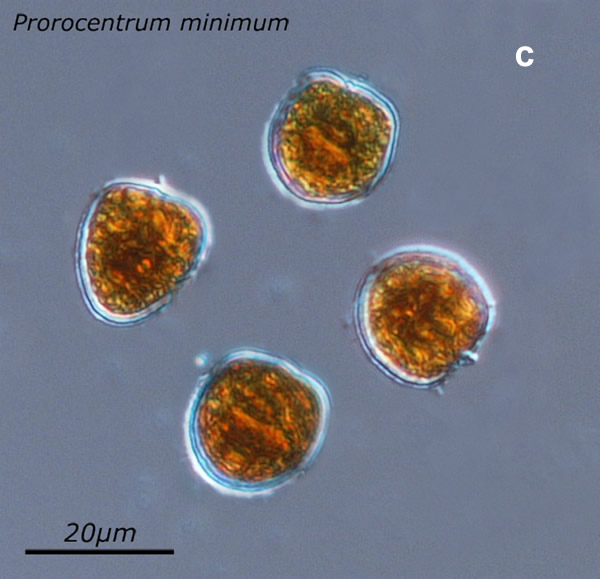
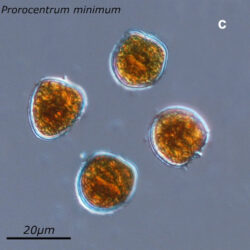
Prorocentrum cordatum, (Ostenfeld) J.D.Dodge, 1975
NUTRITION: autotrophic
ENVIRONMENTAL IMPACT: potentially toxic, invasive and can form intense blooms (red tides)
CELL SIZE: 14-19x 16-22µm
LIFE FORM: solitary
ECOLOGY: marine species
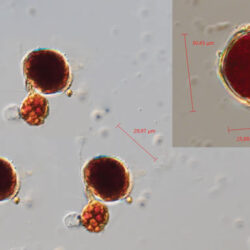
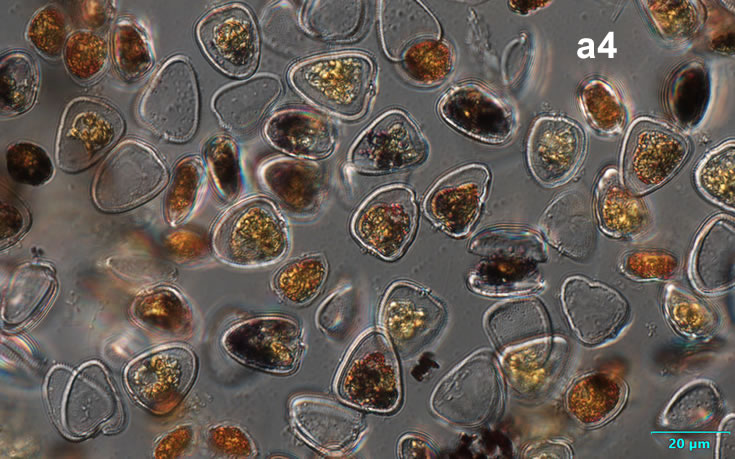
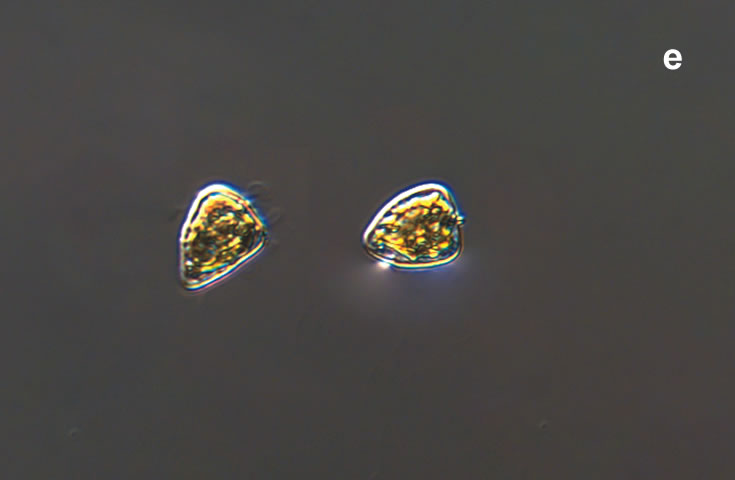
PRESERVATION: September 1999, Lugol (a-a4);
September 2007, living cells (b);
November 2008, Lugol (c);
September 2020, southern Baltic Sea, Lugol (d);
October 2020, Puck Bay, living cells (e).
In September 1999, the largest bloom of P. cordatum was observed in the Gulf of Gdansk (Orlowo pier and Gdynia harbor**). At that time, the biomass was 19 000 µg/l, and the abundance was 15,5 million cells in 1 liter of water. While the annual average biomass usually does not exceed 60 µg/l and abundance 18 000 cells/l.
P. cordatum produces hepatotoxic and diarrhea-inducing toxins. They are the greatest threat to mollusks, but have the potential to affect human health. In the Gulf of Mexico and off the coast of Japan, the species has been responsible for mass shellfish die-offs. No such cases have been reported in the Baltic Sea.
*Olenina, I., Wasmund, N., Hajdu, S., Jurgensone, I., Gromisz, S., Kownacka, J., et al., (2010). Assessing impacts of invasive phytoplankton: the Baltic Sea case. Mar. Pollut. Bull. 60 (10):1691–1700. http://dx.doi.org/10.1016/j.marpolbul.2010.06.046.
**Witek B., Pliński M., 2005. The first recorded bloom of Prorocentrum minimum (Pavillard)Schiller in the coastal zone of the Gulf of Gdańsk. Oceanologia, (42) 1:29-36